Electrocatalytic Nitrate Reduction
 Algae bloom in Michigan, USA5
Algae bloom in Michigan, USA5
Humans contribute over 108 tonnes of reactive nitrogen to the environment each year, largely from fertilizer runoff and industrial processes.1 A major consequence is that aqueous nitrate (NO3–) is one of the most widespread water pollutants.2,3 Consuming nitrate has been linked to infant methemoglobinemia (“blue baby syndrome”), childbirth complications, and ovarian cancer.2,4 High nitrate levels in lakes and oceans also cause mass death of aquatic life through eutrophication.5 Finding an effective strategy to balance the nitrogen cycle is a National of Academy of Engineering grand challenge.6 Concentrated nitrate also exists in low-level nuclear waste,7 and could facilitate spent fuel recovery if removed from the waste stream.8
The electrocatalytic nitrate reduction reaction (NO3RR) is a promising strategy for aqueous denitrification. It converts nitrates to benign or valuable products, such as nitrogen gas (N2) or ammonia (NH3). Unlike thermocatalytic, physical, or biological denitrification, the NO3RR does not require additional chemicals or generate a secondary waste stream.9 This makes NO3RR approaches desirable for modular, decentralized applications. A technoeconomic analysis of low-level nuclear waste found that using the NO3RR to convert nitrate to NH3 and N2 gas could be cost-competitive if a sufficiently stable, active, and selective catalyst exists.10 However, researchers have yet to identify such an electrocatalyst.
My research focuses on discovering active, selective, and stable electrocatalysts to promote NO3RR. I am currently studying bimetallic alloys of earth-abundant metals as well as metal sulfides for this reaction, in collaboration with experimental researchers in the Nirala Singh lab at University of Michigan. Recent projects include an examination of how PtRu alloy composition affects catalyst activity as well as whether metal sulfides are resistant to halide poisoning.
PtRu Alloys
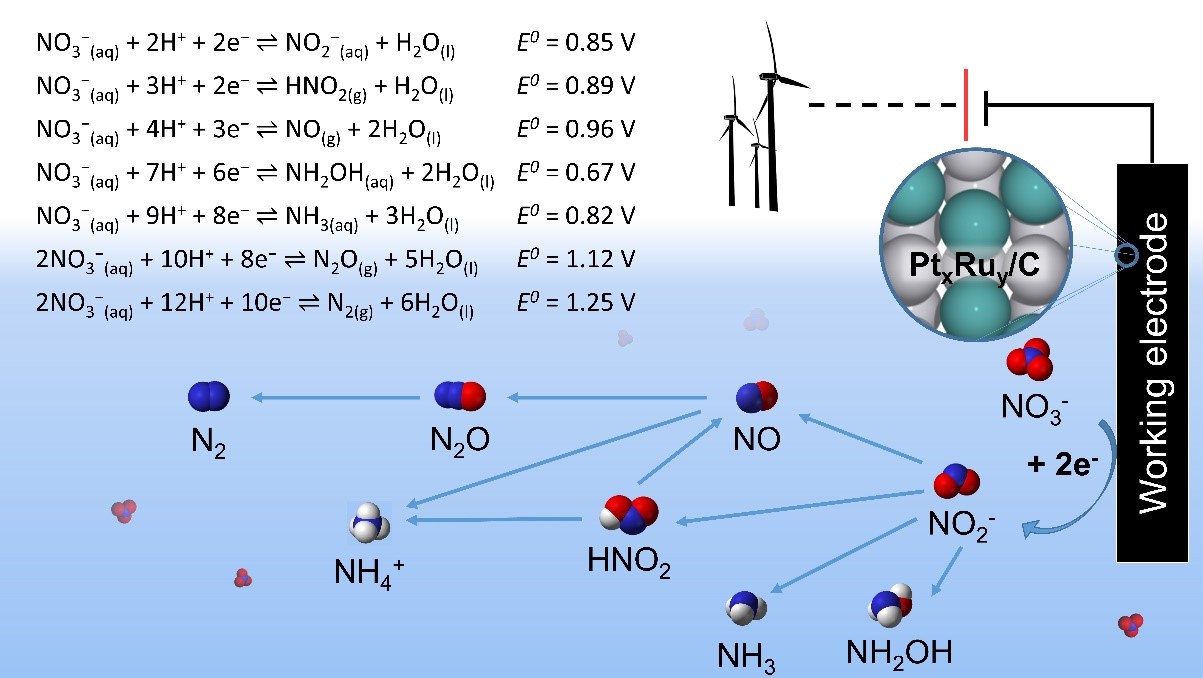
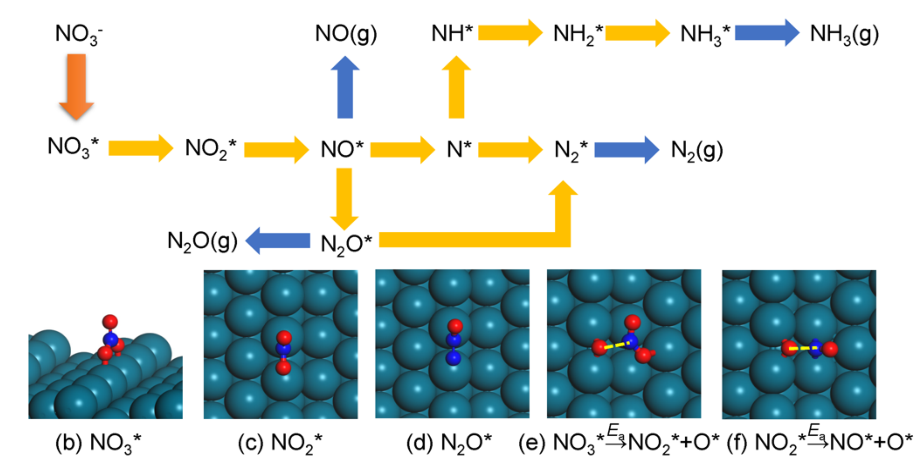
The electrocatalytic nitrate reduction reaction (NO3RR) converts nitrate (NO3–) ions in water to benign or valuable products, such as nitrogen (N2) gas, ammonia (NH3), hydroxylamine (NH2OH), or ammonium nitrate (NH4NO3).11 The rate of conversion and selectivity towards each product depends on a complex reaction mechanism and operating conditions such as solution pH and applied cell voltage. On transition metal surfaces at moderately low (< 1 M) nitrate concentrations and, NO3RR follows a reaction mechanism involving dissociation of surface-bound NO3– to other surface-bound nitrogen intermediates, as well as surface reaction between these intermediates and surface-bound H.12 This mechanism appears below:13
A major challenge in commercializing aqueous-phase electrocatalytic denitrification is the discovery of a sufficiently active, selective, and stable electrocatalyst. Such an electrocatalyst must also attain high activity and selectivity at relatively low (< 1 V) overpotentials.
Of the pure transition metals, Rh is the most active towards NO3RR, as the proposed rate-limiting step (dissociation of NO3* to NO2* and O*) requires a high coverage of NO3, and Rh is able to bind NO3 to its surface more strongly than other metals do. However, Rh is very expensive (~$8,300/oz) and rare, so it is not a practical choice for widespread denitrification applications.
Previous and recent studies have investigated the performance of bimetallic alloys for nitrate reduction and have demonstrated that alloy catalysts can often achieve performance that is better than either pure metal alone. This is accomplished through ligand, strain, and ensemble effects.14 For example, alloying Pt and Sn helps increase the rate of the NO3RR rate-limiting step (nitrate dissociation) and also makes the reaction more selective toward producing hydroxylamine.15 Additionally, a Cu50Ni50 alloy was shown to be six times as active as pure Cu at 0 V vs. RHE.16 It was also shown that the NO3RR activity on the CuNi alloy is a function of the alloy composition, and that there exists an optimal composition that maximizes activity.
Our previous computational work13 predicted that Pt3Ru is a highly active alloy for NO3RR. Our research around PtRu alloys explores whether this is true, and whether NO3RR activity depends on PtRu alloy composition, as it does for CuNi alloys. Preliminary experimental and computational studies suggest that NO3RR activity on PtxRuy alloys is indeed a function of alloy composition, and is maximized at an approximate composition of Pt78Ru22. We are also interested in investigating
- how PtRu alloy composition impacts the selectivity of the reaction towards one or more products,
- which step in the reaction is rate-determining at a variety of alloy compositions and potentials,
- the stability of PtRu alloys in acidic media during long periods of operation, and
- the denitrification cost per gram of catalyst or per mole of nitrate consumed.
The answers to these questions will inform catalyst design rules and reveal insight about which denitrification applications are most appropriate for PtRu alloy electrocatalysts. Our findings are also relevant to other aqueous-phase reactions beyond nitrate reduction.
Metal sulfides
One understudied aspect of heterogeneous catalyst discovery is poison resistance. Poisoning occurs when substances other than reactants compete for and/or block active sites on the catalyst surface, preventing the catalyst from carrying out the desired reaction, as shown in this figure:17
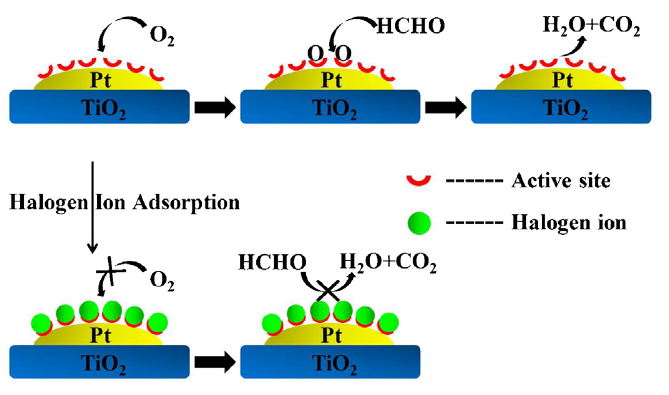
In the context of electrocatalytic nitrate reduction (NO3RR), poisoning can be addressed through upstream preprocessing. However, many catalysts can be poisoned with even trace amounts of contaminants in the reactor feed,18 and upstream purification methods may not remove enough poison. Finding a catalyst that intrinsically resists poisoning is a more robust solution.
My research focuses on halide poisoning, which has been extensively studied in the experimental electrochemical literature as well as in electrochemistry applications (such as PEM fuel cells).18 Halide poisoning is observed experimentally and predicted computationally for many pure metals. A DFT ab initio thermodynamics study found that Cl– was likely to achieve surface coverages of at least 1/3 monolayer in the potential range for nitrate reduction to NH3 and N2.19 Additionally, voltammetry experiments on Pt electrodes show that even trace amounts of Cl– ions (e.g., less than 100 μM) drastically reduce current.20
Metal sulfides (MxSy) are a class of chalcogen compounds which have been shown to resist halide poisoning in some reactions. A mixed-metal sulfide (Co0.4Ru0.6S2) exhibited hydrogen evolution reaction (HER) activity similar to that of Pt in the presence of Br–, yet maintained a stable reaction current while Pt was continuously deactivated.21 Rh sulfides (RhxSy) were also tested against Pt electrodes in a electrolytic cell, where RhxSy was found to maintain moderate HER current long after Pt was completely deactivated.22 Metal sulfides also show promise for enhancing the figures of merit for NO3RR. An oxo-MoS2 catalyst was shown to reduce NO2– to with a selectivity of 13.5% Faradaic efficiency under neutral pH conditions, which is the highest N2 selectivity reported for a metal sulfide electrocatalyst.23 This combination of known halide poison resistance and possible selectivity preference, along with the fact that Rh is the most active transition metal for NO3RR, motivates my current study of RhxSy electrocatalysts.
I am interested in computationally investigating the rate and selectivity of NO3RR on a RhxSy catalyst surface models, focusing on Cl– as a model poison. As RhxSy catalysts have received very little study about halide poison resistance for NO3RR in acidic media, a primary goal is to quantify the degree to which Cl– decreases the reaction rate or changes the selectivity of desirable products such as NH3 and N2. Another important goal is to elucidate the active site for NO3RR on RhxSy surface models through selective poisoning and defect experiments. These insights will help determine whether there exists a selective, active, and stable NO3RR electrocatalyst that is also poison-resistant to one of the most common wastewater poisons. It will also inform design guidelines about how to maximize poison resistance for metal sulfide catalysts in aqueous-phase reactions beyond nitrate reduction.
-
N. Gruber, J.N. Galloway, An Earth-system perspective of the global nitrogen cycle, Nature. 451 (2008) 293–296. https://doi.org/10.1038/nature06592. ↩︎
-
S. Garcia-Segura, M. Lanzarini-Lopes, K. Hristovski, P. Westerhoff, Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications, Appl. Catal. B Environ. 236 (2018) 546–568. https://doi.org/10.1016/j.apcatb.2018.05.041. ↩︎ ↩︎
-
J. Martínez, A. Ortiz, I. Ortiz, State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates, Appl. Catal. B Environ. 207 (2017) 42–59. https://doi.org/10.1016/j.apcatb.2017.02.016. ↩︎
-
B.T. Nolan, K.J. Hitt, B.C. Ruddy, Probability of Nitrate Contamination of Recently Recharged Groundwaters in the Conterminous United States, Environ. Sci. Technol. 36 (2002) 2138–2145. https://doi.org/10.1021/es0113854. ↩︎
-
J. Erickson, Fishing in greener waters: Understanding the impact of harmful algal blooms on Lake Erie anglers, Univ. Mich. News. (2018). https://news.umich.edu/fishing-in-greener-waters-understanding-the-impact-of-harmful-algal-blooms-on-lake-erie-anglers/ (accessed May 3, 2019). ↩︎
-
National Academy of Engineering, Manage the Nitrogen Cycle, NAE Gd. Chall. Eng. (2018). http://www.engineeringchallenges.org/challenges/nitrogen.aspx (accessed November 27, 2018). ↩︎
-
Tian Kuo, Benson Craig H., Tinjum James M., Chemical Characteristics of Leachate in Low-Level Radioactive Waste Disposal Facilities, J. Hazard. Toxic Radioact. Waste. 21 (2017) 04017010. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000361. ↩︎
-
S. Blair, Electrochemical Denitrification of Nuclear Wastewater, (2018). http://large.stanford.edu/courses/2018/ph241/blair2/ (accessed December 5, 2019). ↩︎
-
D. Xu, Y. Li, L. Yin, Y. Ji, J. Niu, Y. Yu, Electrochemical removal of nitrate in industrial wastewater, Front. Environ. Sci. Eng. 12 (2018). https://doi.org/10.1007/s11783-018-1033-z. ↩︎
-
J.O. Bockris, J. Kim, Electrochemical treatment of low-level nuclear wastes, J. Appl. Electrochem. 27 (1997) 623–634. https://doi.org/10.1023/A:1018419316870. ↩︎
-
M. Duca, M.T.M. Koper, Powering Denitrification: The Perspectives of Electrocatalytic Nitrate Reduction, Energy Environ. Sci. 5 (2012) 9726–9742. https://doi.org/10.1039/C2EE23062C ↩︎
-
G.E. Dima, A.C.A. de Vooys, M.T.M. Koper, Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions, J. Electroanal. Chem. 554–555 (2003) 15–23. https://doi.org/10.1016/S0022-0728(02)01443-2 ↩︎
-
J.-X. Liu, D. Richards, N. Singh, B.R. Goldsmith, Activity and Selectivity Trends in Electrocatalytic Nitrate Reduction on Transition Metals, ACS Catal. 9 (2019) 7052–7064. https://doi.org/10.1021/acscatal.9b02179 ↩︎ ↩︎
-
H. Li, K. Shin, G. Henkelman, Effects of ensembles, ligand, and strain on adsorbate binding to alloy surfaces, J. Chem. Phys. 149 (2018) 174705. https://doi.org/10.1063/1.5053894 ↩︎
-
J. Yang, Y. Kwon, M. Duca, M.T.M. Koper, Combining Voltammetry and Ion Chromatography: Application to the Selective Reduction of Nitrate on Pt and PtSn Electrodes, Anal. Chem. 85 (2013) 7645–7649. https://doi.org/10.1021/ac401571w ↩︎
-
Y. Wang, A. Xu, Z. Wang, L. Huang, J. Li, F. Li, J. Wicks, M. Luo, D.-H. Nam, C.-S. Tan, Y. Ding, J. Wu, Y. Lum, C.-T. Dinh, D. Sinton, G. Zheng, E.H. Sargent, Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption, J. Am. Chem. Soc. (2020). https://doi.org/10.1021/jacs.9b13347. ↩︎
-
X. Zhu, B. Cheng, J. Yu, W. Ho, Halogen poisoning effect of Pt-TiO2 for formaldehyde catalytic oxidation performance at room temperature, Appl. Surf. Sci. 364 (2016) 808–814. https://doi.org/10.1016/j.apsusc.2015.12.115. ↩︎
-
F. Juarez, P. Quaino, E. Colombo, E. Santos, M.N. Jackson, W. Schmickler, Why are trace amounts of chloride so highly surface-active?, J. Electroanal. Chem. 847 (2019) 113128. https://doi.org/10.1016/j.jelechem.2019.05.010. ↩︎ ↩︎
-
F. Gossenberger, T. Roman, A. Groß, Equilibrium coverage of halides on metal electrodes, Surf. Sci. 631 (2015) 17–22. https://doi.org/10.1016/j.susc.2014.01.021. ↩︎
-
G. Horányi, E.M. Rizmayer, Role of adsorption phenomena in the electrocatalytic reduction of nitric acid at a platinized platinum electrode, J. Electroanal. Chem. Interfacial Electrochem. 140 (1982) 347–366. https://doi.org/10.1016/0022-0728(82)85178-4. ↩︎
-
A. Ivanovskaya, N. Singh, R.-F. Liu, H. Kreutzer, J. Baltrusaitis, T. Van Nguyen, H. Metiu, E. McFarland, Transition Metal Sulfide Hydrogen Evolution Catalysts for Hydrobromic Acid Electrolysis, Langmuir. 29 (2013) 480–492. https://doi.org/10.1021/la3032489. ↩︎
-
N. Singh, S. Mubeen, J. Lee, H. Metiu, M. Moskovits, E.W. McFarland, Stable electrocatalysts for autonomous photoelectrolysis of hydrobromic acid using single-junction solar cells, Energy Environ. Sci. 7 (2014) 978–981. https://doi.org/10.1039/C3EE43709D. ↩︎
-
D. He, Y. Li, H. Ooka, Y.K. Go, F. Jin, S.H. Kim, R. Nakamura, Selective Electrocatalytic Reduction of Nitrite to Dinitrogen Based on Decoupled Proton–Electron Transfer, J. Am. Chem. Soc. 140 (2018) 2012–2015. https://doi.org/10.1021/jacs.7b12774. ↩︎